Approach aims to improve patient outcomes, save money, engage physicians
By JULIE MINDA
When many health care providers contract for medical devices, they do so without head-to-head comparisons of similar products from different manufacturers. And many systems do not have a standard process for securing extensive clinician input before purchasing, according to Dr. Robert Lerman, vice president of medical effectiveness for San Francisco-based Dignity Health.
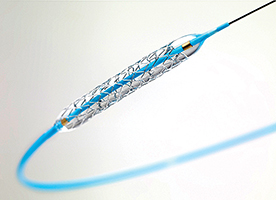
The Medtronic Resolute Integrity drug-eluting stent, above, and the Supera peripheral stent from Abbott, at far right, are two of the devices approved for purchase through the SharedClarity review process.
To forge a well-informed, value-driven approach to contracting, Dignity Health, three other large health systems and United-
Healthcare are partnering on a joint venture company called SharedClarity. The young company uses a variety of methods of study, including a thorough clinical review of existing scientific literature and input from surgeons, to surmise which implanted medical devices perform best over the long term for patients. SharedClarity then contracts for those top products on behalf of its owner companies and other health care providers.
The other partners in SharedClarity are Downers Grove, Ill.-based Advocate Health Care; Dallas-based Baylor, Scott and White; and Flint, Mich.-based McLaren Health Care.
A key goal, and what the partners hope will be the main ingredient that sets this initiative apart, is to create a proprietary database that marries claims data from UnitedHealthcare and clinical outcome information from the health system partners. That will yield longitudinal patient outcome information that will inform SharedClarity's head-to-head comparisons of medical devices, said Lerman. Measures of effectiveness will vary by device.
SharedClarity members are still determining how best to create and use this type of database, since there is rapid cycle improvement of medical devices, and so products implanted in patients even in the recent past may not be the same version of products available at the time of study, according to Lerman.
But the partners see great potential to use that shared data to fine-tune care protocols to improve patient outcomes, said Rich Roth, chief strategic innovation officer for Dignity Health. For instance, they could use data to determine whether a patient who received "prehab," or therapy prior to a procedure, fared better with the implantation of a device than a control group. The partners eventually want to pull data and get input from clinicians across the care continuum.
Joint venture
Roth said that Dignity and UnitedHealthcare developed the SharedClarity concept several years ago while brainstorming ways to partner to improve patient quality while also containing costs. He explained, "We saw that (the medical device industry) was like a black box, and we saw an opportunity to shine the light, relative to the effectiveness and value" of certain devices.
Launched in 2013, the private, for-profit SharedClarity is based in Phoenix and employs 11. Each of the five joint venture partners has made a financial investment in SharedClarity and has representation on its board of managers as well as on all of SharedClarity's clinical review teams; contracting teams; and on its new technology, quality and ethics, strategy and customer advisory councils. SharedClarity
President Mark West would not disclose the amount — or percentage — of each owner's investment. He said there is no majority owner.
The partners are recruiting an additional four health systems to share ownership. SharedClarity also is recruiting customers, or health systems that would not have an ownership stake but that would — like all the other health systems — pay a fee to participate in the purchasing contracts negotiated by SharedClarity. Lerman said SharedClarity uses the collective buying power of its members to get favorable pricing on medical devices.
High-impact priorities
Within about the next five years, SharedClarity plans to complete its analysis and contracting process for 30 medical devices that West characterizes as "high-tech, high-cost and high-impact, meaning they have an impact on the patient's lifestyle because they are implanted and stay in you." These include stents, defibrillators, pacemakers, knee and hip implants and heart valves. So far, the partners have completed the evaluation process for bare metal cardiac stents, drug-eluting cardiac stents and peripheral arterial stents. They are currently evaluating contrast agents for medical imaging, surgical hernia mesh and cardiac pacemakers and implantable defibrillators, according to information on SharedClarity's website.
For each device category, Shared-Clarity assembles a clinical review team that includes SharedClarity representatives as well as clinicians from each owner health system. By engaging the health care systems' clinicians in a more extensive and standardized review of devices than is typical, SharedClarity will glean practical insights that will inform device comparison, said Lerman.
"We're focusing first on the clinical and comparative effectiveness data — objective data — and the experience and preferences of our experts," the clinicians who implant the devices, Lerman said. "And then we are moving forward and looking at the contracts from a cost standpoint. We're putting the clinical considerations first."
Exhaustive review
The review process is exhaustive. A clinical review team that just completed an evaluation of contrast media used in medical imaging compared 23 brands. Team members read more than 5,000 pages of medical literature, including studies and research from device manufacturers and from academicians funded independently or by manufacturers. It solicited input from clinicians representing six medical disciplines within the owner health systems, according to West.
Once a review team reaches a consensus on the highest value devices in a category, SharedClarity will contract for equipment or products, which could be sourced from multiple manufacturers, according to Lerman. If a review team can't reach consensus, it may recommend that SharedClarity fund an independent study of devices in that category, he said, noting that so far, such an independent study has not been necessary.
West, Roth and Lerman said SharedClarity is too early in its work to share information on cost savings on patient care or on the devices themselves. But, they said, SharedClarity members already are seeing savings achieved on device purchases through contracting.
More importantly, Roth said, "Under health care reform, multiple organizations need to work together, and to create value. This is a great example of a payer and a group of providers tackling an issue of concern to all of us."